

The length of \(r\) is the distance between the proton and the electron, and the direction of \(r\) and the direction of \(r\) is given by the orientation of the vector pointing from the proton to the electron. This reduced particle is located at \(r\), where \(r\) is the vector specifying the position of the electron relative to the position of the proton.
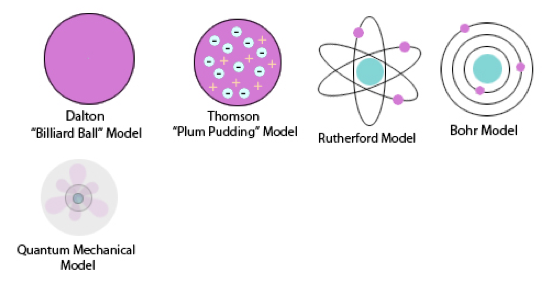
Werner Heisenberg was a German physicist and philosopher who discovered a way to formulate quantum mechanics.He contributed to the atomic theory by including. This atomic model is known as the quantum mechanical model of the atom. The hydrogen atom, consisting of an electron and a proton, is a two-particle system, and the internal motion of two particles around their center of mass is equivalent to the motion of a single particle with a reduced mass. The Schrödinger-Lohe model consists of wave functions interacting with each other, according to a system of Schrödinger equations with a specific coupling such that all wave functions evolve on the L 2 unit ball. Schrdinger used mathematical equations to describe the likelihood of finding an electron in a certain position.


 0 kommentar(er)
0 kommentar(er)
